doi: 10.56294/dm202212
SYSTEMATIC REVIEW
Can we administer Acetazolamide in patients with heart failure? A systematic review
¿Podemos administrar Acetazolamida en pacientes con insuficiencia cardiaca? Revisión sistemática
Maria Eduarda Santos Luna1 ![]() *
*
1Universidad Abierta Interamericana, Facultad de Medicina y Ciencias de la Salud, Carrera de Medicina. Ciudad Autónoma de Buenos Aires, Argentina.
Citar como: Santos Luna ME. Can we administer Acetazolamide in patients with heart failure? A systematic review. Data & Metadata. 2022;1:12. https://doi.org/10.56294/dm202212
Submitted: 24-10-2022 Revised: 29-11-2022 Accepted: 23-12-2022 Published: 24-12-2022
Editor: Prof.
Dr. Javier González Argote ![]()
ABSTRACT
Introduction: heart failure (HF) is a clinical syndrome characterized by a variety of symptoms and signs due to structural and/or functional abnormalities of the heart leading to decreased heart rate, cardiac output and/or increased intracardiac pressures. Studies suggest that acetazolamide could improve the efficacy of ASA diuretics, which could lead to improved natriuresis and diuresis.
Aims: to evaluate the effectiveness of acetazolamide in improving congestion symptoms in patients with Heart Failure.
Methods: a systematic review will be carried out, following the PRISMA methodology, which will include a search in Pubmed, Scopus, Web of Science databases without time restriction.
Results: 5 studies were included, of which 4 were clinical trials.
Conclusions: after comprehensive and detailed analysis of the included studies, we found limited evidence suggesting that the use of acetazolamide may be effective in the treatment of heart failure, especially as additional or complementary therapy to other treatments. However, it is important to keep in mind that the results of the pilot studies are limited and that more research is required to fully evaluate the efficacy and safety of acetazolamide in the treatment of heart failure. The use of acetazolamide in patients with heart failure may be controversial and requires careful evaluation of clinical risks and benefits before it is considered as a treatment.
Keywords: Heart Failure; Acetazolamide; Congestion; Diuretics; Pharmacological Action.
RESUMEN
Introducción: la insuficiencia cardíaca (IC) es un síndrome clínico caracterizado por una variedad de síntomas y signos por anomalías estructurales y/o funcionales del corazón que provocan una disminución de la frecuencia cardiaca, gasto cardíaco y/o aumento de las presiones intracardiacas. Estudios plantean que la acetazolamida podría mejorar la eficacia de los diuréticos de ASA, lo que podría conducir a una mejor natriuresis y diuresis.
Objetivos: evaluar la efectividad de la acetazolamida en el mejoramiento de los síntomas de congestión en pacientes con Insuficiencia Cardíaca.
Métodos: se realizará una revisión sistemática, siguiendo la metodología PRISMA, que incluirá la búsqueda en las bases de datos Pubmed, Scopus, Web of Science sin restricción temporal.
Resultados: se incluyeron 5 estudios, de los cuales 4 eran ensayos clínicos.
Conclusiones: luego del análisis integral y pormenorizado de los estudios incluidos, se encontró evidencia limitada que sugieren que el uso de acetazolamida puede ser efectivo en el tratamiento de la insuficiencia cardíaca, especialmente como terapia adicional o complementaria a otros tratamientos. Sin embargo, es importante tener en cuenta que los resultados de los estudios piloto son limitados y que se requiere más investigación para evaluar plenamente la eficacia y la seguridad de acetazolamida en el tratamiento de la insuficiencia cardíaca. El uso de acetazolamida en pacientes con insuficiencia cardíaca puede ser controvertido y requiere una evaluación cuidadosa de los riesgos y los beneficios clínicos antes de ser considerado como un tratamiento.
Palabras Clave: Insuficiencia Cardíaca; Acetazolamida; Congestión; Diuréticos; Acción Farmacológica.
INTRODUCTION
Heart failure (HF) is a clinical syndrome characterized by various symptoms and signs due to structural and/or functional abnormalities of the heart that cause a decrease in heart rate, cardiac output, and/or increased intracardiac pressures.(1)
Patients with heart failure are generally divided into two groups based on left ventricular systolic function: heart failure with reduced ejection fraction (HFrEF) with a left ventricular ejection fraction less than 40 % or heart failure with a left ventricular ejection fraction less than 40 % ejection fraction (HFpEF) with left ventricular ejection fraction greater than 50 %.(2)
Many diseases can cause heart failure, but coronary heart disease and high blood pressure are the leading causes, ahead of dilated cardiomyopathy, hypertrophic cardiomyopathy or restrictive cardiomyopathy, and valve diseases.(3)
Symptoms of heart failure include dyspnea (difficulty breathing) and fatigue, which may be accompanied by typical signs of congestion, such as rales (abnormal crackling sounds), lower limb edema, and/or distended jugular veins.(4)
Structural or functional heart disease is a prerequisite for HF. It includes a variety of acute or chronic heart diseases that lead to the activation of several pathophysiological pathways (initially adaptive and eventually maladaptive), which neutralize the negative effect of heart failure on the oxygenation of peripheral tissues, but that ultimately can also lead to systemic congestion, ventricular congestion, organ remodeling, and dysfunction.(4)
Diastolic or systolic dysfunction of the left ventricle results in increased preload and afterload, leading to pulmonary congestion. Fluid retention and redistribution result in systemic congestion, ultimately leading to organ dysfunction due to hypoperfusion.(4)
Systemic congestion significantly impacts the clinical presentation of most HF patients and is an important determinant of multiorgan dysfunction in HF. The pathophysiology of HF is heterogeneous, as the characteristics of the underlying heart disease strongly influence it.(5)
Given the suspicion of HF, an electrocardiogram is indicated for all patients. The doses of BNP or NT-pro-BNP are of great help in the diagnosis. Factors such as the patient's age, creatinine, and other diseases must be considered for their interpretation. Another study of great importance here is echocardiography, which makes it possible to measure LVEF, assess hemodynamics, and search for etiology.(2,3)
Treatment should be based on etiology, ejection fraction, and symptom presentation. Its main objective is to reduce the overload of the heart and the progression of remodeling, decrease the pulmonary or systemic congestive state, and counteract neurohormonal alterations.(2)
Within the classes of drugs most used for treating heart failure, we associate diuretics (drugs of great importance for the signs and symptoms of congestion) with angiotensin-converting enzyme (ACE) inhibitors or receptor blockers of angiotensin II (ARA2), if there is intolerance and beta-blockers. But that does not mean that all patients receive this treatment. Everything will depend on the presentation of the disease and the underlying pathology.(3)
Carbonic anhydrase (CA) is a zinc-dependent metalloenzyme that has a vital role in the reversible catalyzation of CO2 to form HCO3- and H+. There are five families of proteins, and the AC-a family is the one you find in humans. This family is divided into four subgroups and fourteen isoforms. Its wide cellular distribution gives it different functionalities.(6)
By catalyzing the simple but essential hydration of CO2 to bicarbonate and protons, AC participates in critical steps in the life cycle of many organisms, including eukaryotes, bacteria, and archaea.(7)
Classic carbonic anhydrase inhibitor (CAI) drugs produce a competitive type of inhibition by binding to the zinc molecule. They strongly inhibit CAs belonging to most families, not just enzymes in the class.(7)
Side effects of many of the first and second-generation CAI compounds (including metabolic acidosis, kidney stones, bone loss, etc.) are due to the potent inhibition of all CA isomers, not just the target.(7)
The most important classes of CAI are the sulfonamides, with several compounds such as acetazolamide, methazolamide, etoxzolamide, sulthiame, dichlorophenamide, dorzolamide, brinzolamide, sulpiride, and zonisamide, which are used clinically as diuretics, antiglaucoma drugs, and antiepileptics.(8)
The main reason that patients with acute heart failure seek urgent care is because of the signs and symptoms of congestion. This happens because the increase in neurohumoral activation in HF with congestion increases sodium and water absorption. Current guidelines recommend using loop diuretics to relieve the signs and symptoms of fluid overload.(9)
Acetazolamide is a diuretic that inhibits carbonic anhydrase and works by preventing sodium reabsorption in the proximal tubules of the nephron. By itself, acetazolamide's diuretic and natriuretic abilities are poor, but when combined with ASA diuretics, they help relieve congestion and make the combination more effective.(10)
Studies suggest that acetazolamide could improve the efficacy of ASA diuretics, which could lead to better natriuresis and diuresis. This would then translate into better clinical outcomes, a shorter hospital stay, and better quality of life.(11,12)
On the other hand, unabsorbed chloride in the proximal tubule will reach the dense macular cells at the end of the loop of Henle, stopping renin release and neurohumoral activation. Finally, endogenous natriuretic peptides, mainly in the distal nephron, can return to work.(13)
Bearing in mind that there is a great need to demonstrate, based on the available evidence, the effectiveness of new interventions that promote diuresis effectively and safely and improve the success of decongestion in AHF with volume overload. On the other hand, in the reviewed literature, there are reasons from the pathophysiological point of view which support that acetazolamide increases the efficacy of ASA diuretics in patients with AHF. This investigation will be carried out. The results derived from this work will serve as scientific and theoretical support for using this drug in other areas little known.
Objective: to describe the evidence on using acetazolamide to improve congestion symptoms in patients with Heart Failure.
METHODS
Study design
Research results were synthesized through a systematic review.
This systematic review was governed according to the PRISMA guidelines (preferred reporting items for systematic reviews and meta-analyses).(14)
Study population
Scientific articles on patients with Acute Heart Failure with Acetazolamide treatment as a cardiovascular therapeutic strategy were included. No time limitation will be made.
Inclusion criteria
• Original articles with IMRyD typology that develop cohort studies, clinical trials, other systematic reviews, and meta-analyses.
Exclusion criteria
• Review Articles, Scientific Letters / Letters to the Editor, Clinical Cases, Editorials, and Original Articles corresponding to Preclinical and Observational Studies.
Sample
A search was carried out in Pubmed, Scopus, and Web of Science without time restriction. Cohort and case-control studies were selected to evaluate the effects of improving congestion symptoms in patients with Acute Heart Failure with Acetazolamide treatment as a cardiovascular therapeutic strategy.
Bibliographic search strategy
Pubmed: “Acetazolamide”[Mesh] AND “Heart Failure”[Mesh].
Scopus: “Acetazolamide” AND “Heart Failure”.
Web of Science: TS=(“Acetazolamide” AND “Heart Failure”)
RESULTS
Three hundred eighty-two references were found, of which 378 were eliminated because they were not empirical articles, did not address the review's objective, or did not have the full text. Finally, four articles were included (figure 1).
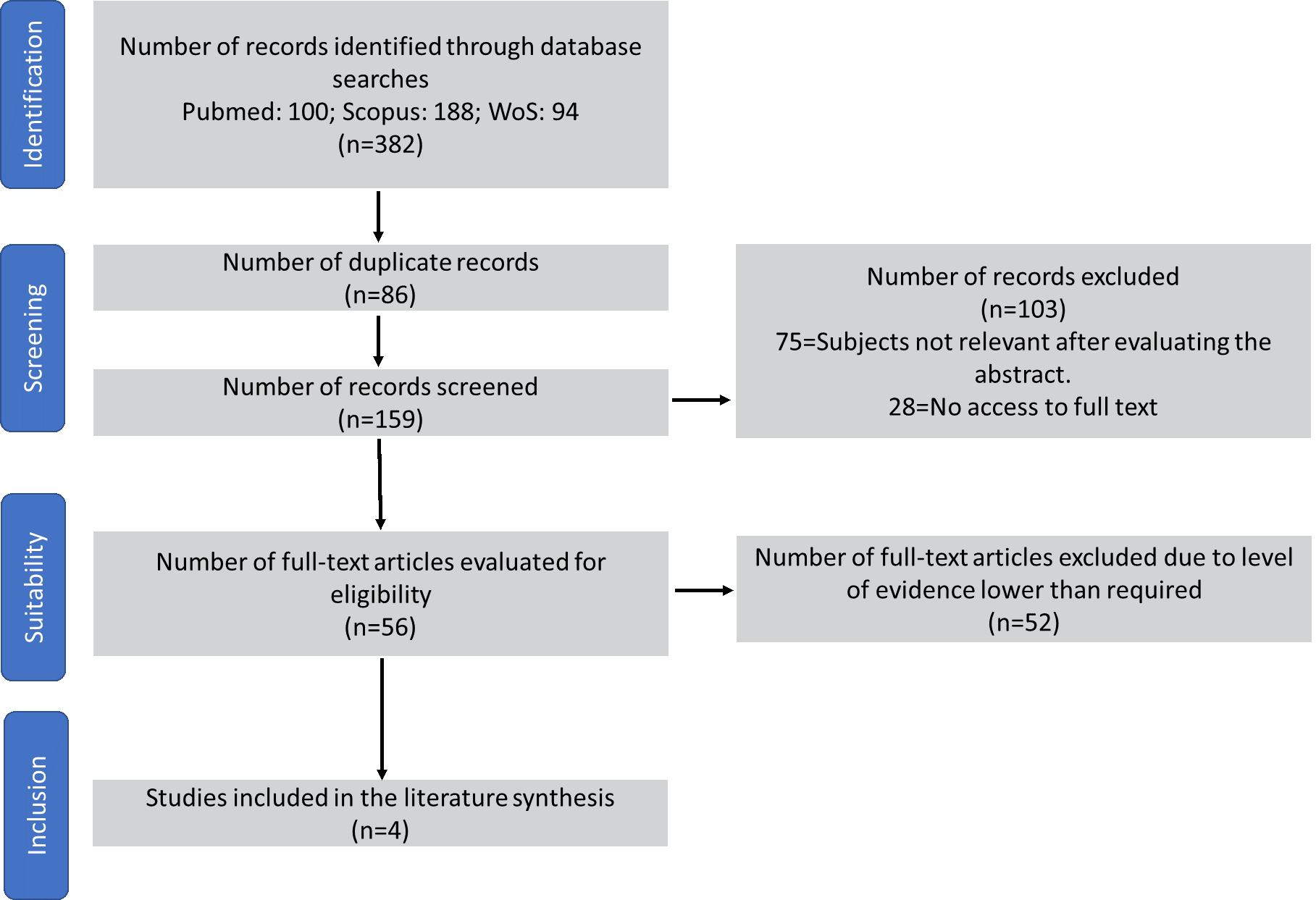
Figure 1. Article selection process according to the PRISMA flowchart
Table 1 shows the main characteristics and results of the articles included in the systematic review.
|
Table 1. Characteristics and main results of incluted studies |
||||||
|
Study |
Country |
Aim |
Treatment |
Type of study |
Sample |
Main results |
|
Kataoka(15), 2019 |
Japan |
To compare the effects of the diuretic acetazolamide with those of conventional diuretics on plasma volume, serum electrolytes, and renal function in patients with acute decompensated heart failure (HF). |
Acetazolamide (Diamox) vs. conventional diuretics |
Retrospective observational study |
N= 26 GC= 13 GE=13 |
Both groups exhibited a reduction in equivalent body weight and resolution of HF-related signs after each diuretic treatment, but acetazolamide treatment preserved plasma volume and renal function compared to conventional diuretics. |
|
Imiela et al.(16), 2017 |
Poland |
The aim of this study was to determine the diuretic effect of acetazolamide in patients with exacerbations of chronic heart failure, in addition to their stable diuretic treatment. |
Acetazolamide associated with stable diuretic. |
Prospective, randomized, unblinded, single-center, randomized study. |
N= 20 GC= 10 GE= 10 |
The results of this pilot study suggest that the addition of acetazolamide to other stable diuretic drugs in hospitalized patients with exacerbation of heart failure may produce an additional diuretic effect and relief of dyspnea. |
|
Mullens et al.(17), 2022 |
Belgium |
To investigate whether acetazolamide can improve the efficacy of loop diuretics, leading to greater and more rapid decongestion in patients with acute decompensated heart failure with volume overload. |
Intravenous acetazolamide (500 mg once a day) or placebo added to standardized intravenous loop diuretics (at a dose equivalent to twice the maintenance oral dose). |
Multicenter, parallel-group, double-blind, randomized, placebo-controlled trial. |
N=519 GC= 256 (acetazolamide) GE=259 (placebo) |
The addition of acetazolamide to loop diuretic therapy in patients with acute decompensated heart failure resulted in a higher incidence of successful decongestion. |
|
Verbrugge et al.(18), 2019 |
Belgium |
To investigate the effects of acetazolamide on natriuresis, decongestion, renal function, and neurohumoral activation in acute heart failure (AHF). |
Single-blind combination therapy with acetazolamide and low-dose loop diuretics vs. low-dose loop diuretics versus monotherapy with high-dose loop diuretics; and open-label oral and open-label oral spironolactone administered in advance of discharge. in advance vs. at discharge. |
Randomized, prospective, investigator-driven, prospective study investigator |
N= 34 GC= 16 (high-dose ASA diuretic monotherapy) GE= 18 (acetazolamide with low-dose ASA diuretic). |
In an AHF population with advanced disease and high risk of diuretic resistance, acetazolamide with low-dose loop diuretics produced similar natriuresis compared with increasing the dose of loop diuretics as monotherapy. |
|
Javaheri(19), 2006 |
Ohio |
To determine the therapeutic efficacy of acetazolamide in central sleep apnea associated with heart failure |
Acetazolamide vs. placebo, taken 1 h before going to bed for six nights. |
Double-blind, placebo-controlled, crossover clinical trial. |
N= 12 |
There were no significant differences in sleep apnea and hypopnea episodes and arterial oxyhemoglobin desaturation when baseline and placebo were compared. However, acetazolamide resulted in a significant reduction in central apneas. |
DISCUSSION
Acetazolamide is a medication that is commonly used to treat disorders such as edema (fluid buildup in the tissues), intracranial hypertension (raised pressure inside the skull), and glaucoma (an eye disease that can cause vision loss). It is a carbonic diuretic, which increases the excretion of carbon dioxide in the urine, increasing the excretion of fluids.
Studies suggest that acetazolamide may be effective in treating heart failure, especially as an add-on or complementary therapy to other treatments. For example, Imiela et al.(16) study found that acetazolamide may be a valuable therapy for patients with chronic heart failure and acute exacerbations. On the other hand, Kataoka(20) compared the effects of acetazolamide and conventional diuretics on plasma volume and renal function and found that acetazolamide may be a promising alternative to traditional diuretics.
Regarding evaluating the efficacy and safety of acetazolamide in treating heart failure, Mullens et al.(17) found that acetazolamide may be effective in treating acute decompensated heart failure with volume overload. Still, the number of patients included in this study was small.
Acetazolamide administration can decrease fluid retention and improve ventricular function in patients with heart failure. As a result, BNP and NT-proBNP levels may decrease, reflecting improved cardiac function, which is reflected in a better EQ-5D result. Administration of acetazolamide reduced plasma volume, which contributes to improving cardiac function by increasing sodium and fluid excretion.
Acetazolamide is a diuretic, which in the case of patients with cardiovascular risk, requires careful evaluation by a physician due to its possible side effects.(18)
Although in previous studies, it was reported that acetazolamide caused electrolyte imbalances and alterations in cardiac function, especially in patients with advanced heart disease, in addition to increasing the burden on the heart and worsening cardiac function, especially in patients with high cardiovascular risk. The studies found in this review, although few, are encouraging based on the improvement of cardiac congestion and renal values.
Beyond these results, it should be noted that before considering the use of acetazolamide, it be closely monitored during treatment for any possible side effects or worsening of cardiac function.
There are several important limitations in the included studies:
1. Small sample size: many studies are pilot studies or case-control studies with a limited number of patients, which may make it difficult to generalize the results to the general population.
2. Short duration of follow-up: some studies only followed patients for a short period, which may make it difficult to assess the long-term effects of acetazolamide.
3. Lack of control group: some studies lack an adequate control group, which may make it difficult to assess the specific effects of acetazolamide compared to other treatments or placebo.
4. Non-randomized design: some studies have a non-randomized design, which increases the risk of bias and may make it difficult to assess the results objectively.
5. Lack of standardized follow-up: some studies lack standardized follow-up of patients, which may affect the accuracy of the results.
Considering these limitations when interpreting the results to consider acetazolamide as a potential treatment for heart failure is essential.
CONCLUSIONS
After a comprehensive and detailed analysis of the included studies, limited evidence suggested that acetazolamide may be effective in treating heart failure, especially as an additional or complementary therapy to other treatments. However, it is important to note that the results of the pilot studies are limited, and more research is required to fully assess the efficacy and safety of acetazolamide in treating heart failure. The use of acetazolamide in patients with heart failure may be controversial and requires careful evaluation of the clinical risks and benefits before it is considered a treatment.
REFERENCES
1. Kurmani S, Squire I. Acute Heart Failure: Definition, Classification and Epidemiology. Curr Heart Fail Rep 2017;14:385-92. https://doi.org/10.1007/s11897-017-0351-y.
2. Snipelisky D, Chaudhry S-P, Stewart GC. The Many Faces of Heart Failure. Card Electrophysiol Clin 2019;11:11-20. https://doi.org/10.1016/j.ccep.2018.11.001.
3. Robles Gamboa C. Insuficiencia cardíaca crónica. Medicine - Programa de Formación Médica Continuada Acreditado 2017;12:2100-15. https://doi.org/10.1016/j.med.2017.06.003.
4. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers 2020;6:1-15. https://doi.org/10.1038/s41572-020-0151-7.
5. Sinnenberg L, Givertz MM. Acute heart failure. Trends in Cardiovascular Medicine 2020;30:104-12. https://doi.org/10.1016/j.tcm.2019.03.007
6. Monroy LE, Vargas MPS. Anhidrasa carbónica, nuevas perspectivas. Neumol Cir Torax 2010;69:200-9.
7. McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. Subcell Biochem 2014;75:291-323. https://doi.org/10.1007/978-94-007-7359-2_15.
8. Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709-12. https://doi.org/10.1080/13543776.2018.1523897.
9. Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail 2018;20:1591-600. https://doi.org/10.1002/ejhf.1307.
10. Acar S, Sanli S, Oztosun C, Afsar B, Sag AA, Kuwabara M, et al. Pharmacologic and interventional paradigms of diuretic resistance in congestive heart failure: a narrative review. Int Urol Nephrol 2021;53:1839-49. https://doi.org/10.1007/s11255-020-02704-7.
11. Cimolai N. Acetazolamide and Cardiac Failure. Clin Drug Investig 2018;38:649-50. https://doi.org/10.1007/s40261-018-0653-1.
12. Cimolai N. Acetazolamide and Cardiac Failure. Clin Drug Investig 2018;38:649-50. https://doi.org/10.1007/s40261-018-0653-1.
13. Cuthbert JJ, Bhandari S, Clark AL. Hypochloraemia in Patients with Heart Failure: Causes and Consequences. Cardiol Ther 2020;9:333-47. https://doi.org/10.1007/s40119-020-00194-3.
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Revista Española de Cardiología 2021;74:790-9. https://doi.org/10.1016/j.recesp.2021.06.016.
15. Kataoka H. Acetazolamide as a potent chloride-regaining diuretic: short- and long-term effects, and its pharmacologic role under the ‘chloride theory’ for heart failure pathophysiology. Heart Vessels 2019;34:1952-60. https://doi.org/10.1007/s00380-019-01433-x.
16. Imiela T, Budaj A. Acetazolamide as Add-on Diuretic Therapy in Exacerbations of Chronic Heart Failure: a Pilot Study. Clin Drug Investig 2017;37:1175-81. https://doi.org/10.1007/s40261-017-0577-1.
17. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N Engl J Med 2022;387:1185-95. https://doi.org/10.1056/NEJMoa2203094.
18. Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, et al. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. European Journal of Heart Failure 2019;21:1415-22. https://doi.org/10.1002/ejhf.1478.
19. Javaheri S. Acetazolamide Improves Central Sleep Apnea in Heart Failure. Am J Respir Crit Care Med 2006;173:234-7. https://doi.org/10.1164/rccm.200507-1035OC.
20. Kataoka H. Comparison of Changes in Plasma Volume and Renal Function between Acetazolamide and Conventional Diuretics: Understanding the Mechanical Differences according to the “Chloride Theory”. CRD 2020;145:215-23. https://doi.org/10.1159/000504533.
FINANCING
None.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Maria Eduarda Santos Luna.
Research: Maria Eduarda Santos Luna.
Methodology: Maria Eduarda Santos Luna.
Formal analysis: Maria Eduarda Santos Luna.
Research: Maria Eduarda Santos Luna.
Writing - Original draft: Maria Eduarda Santos Luna.
Writing - Revision and editing: Maria Eduarda Santos Luna.